AstraZeneca’s Decision to Withdraw Amid Surplus of Updated Vaccines.
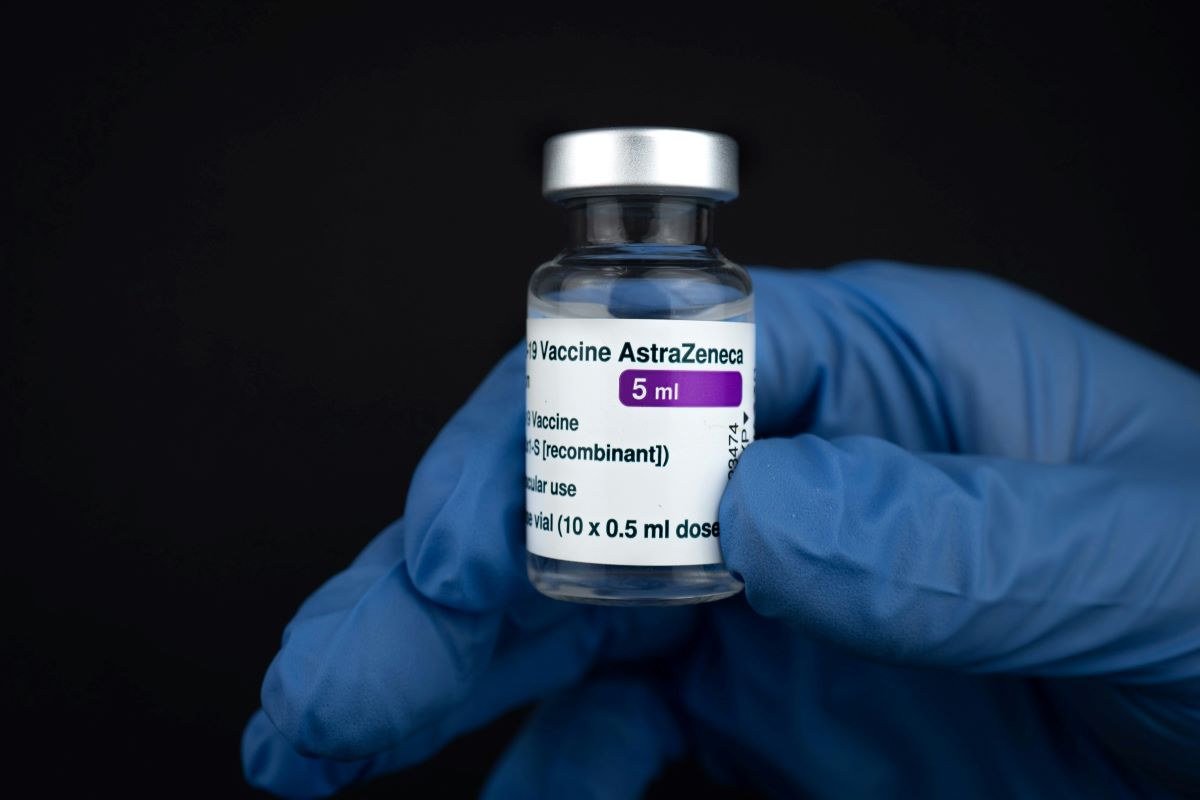
AstraZeneca, a pharmaceutical giant at the forefront of the battle against COVID-19, has recently made a significant decision to withdraw its COVID-19 vaccine from circulation globally. This move comes as a response to what the company describes as a “surplus of available updated vaccines” amid the ongoing pandemic. With the emergence of multiple variant-specific vaccines, the demand for AstraZeneca’s Vaxzevria has diminished, prompting the company to cease its manufacturing and distribution efforts.
Search
Recent Posts:
- OpenAI Brings ChatGPT to be used in WhatsApp: Here’s How It Works and What You Can Do To Use It.
- Realme 14x 5G: A Budget Smartphone With Premium Features.
- Exploring Apple Genmoji: A New Era of Custom Emoji Creation.
- 2024 United States Presidential Election: Donald Trump Declares Victory in 2024 Presidential Election
- Chancellor Olaf Scholz’s Visit to India: Advancing Indo-German Cooperation on Defense, Trade, and Regional Stability.
Implications for Global Health Policy and Legal Landscape.
The decision to withdraw the vaccine highlights the rapidly evolving landscape of the COVID-19 pandemic and the shifting dynamics of vaccine availability and demand. While the AstraZeneca vaccine was once heralded as a critical tool in the fight against the virus, concerns over its safety and efficacy have prompted a reevaluation of its role in vaccination campaigns worldwide.
One of the primary factors contributing to the withdrawal of the AstraZeneca vaccine is the emergence of rare but potentially serious side effects, such as Thrombosis with Thrombocytopenia Syndrome (TTS). Although the causal link between the vaccine and TTS remains uncertain, AstraZeneca has taken proactive steps to address concerns by voluntarily withdrawing its marketing authorization in the European Union and discontinuing production.
The decision to withdraw the AstraZeneca vaccine has sparked debate and legal action, with victims and their families seeking damages estimated to be worth up to £100 million. Allegations of vaccine defectiveness and overstated efficacy have been vehemently denied by the company, setting the stage for a complex legal battle that could have far-reaching implications for the pharmaceutical industry and public health policy.
Safeguarding Vaccine Confidence Amidst Evolving Safety Concerns.
In the wake of the AstraZeneca vaccine withdrawal, questions have arisen regarding the safety and efficacy of COVID-19 vaccines, particularly in regions heavily reliant on the AstraZeneca-produced Covishield. India, for example, has administered over 1.7 billion doses of Covishield as part of the world’s largest vaccination program. Despite concerns over rare side effects such as TTS, medical experts emphasize that the benefits of vaccination far outweigh the risks, and the withdrawal of the AstraZeneca vaccine should not deter individuals from seeking vaccination.
It is important to recognize that the withdrawal of the AstraZeneca vaccine underscores the need for ongoing vigilance and transparency in vaccine safety monitoring. While rare adverse effects such as TTS require prompt medical attention, they should not overshadow the broader benefits of vaccination in curbing the spread of COVID-19 and preventing severe illness and death. As the global vaccination effort continues, it is essential for individuals to remain informed and proactive in their healthcare decisions.
Consulting with healthcare professionals and staying updated on the latest developments can help ensure a safe and effective vaccination experience for individuals and communities worldwide. Additionally, efforts to enhance vaccine safety monitoring and address concerns about vaccine efficacy and side effects are crucial in maintaining public trust and confidence in vaccination programs. By working together and prioritizing public health, we can overcome the challenges posed by the COVID-19 pandemic and emerge stronger and more resilient than ever before.
What is TTS(Thrombosis with Thrombocytopenia Syndrome)?

Thrombosis with Thrombocytopenia syndrome (TTS) is an uncommon yet severe condition linked to specific COVID-19 vaccines, notably adenovirus vector vaccines like the AstraZeneca vaccine and Johnson & Johnson’s Janssen vaccine.
This syndrome typically manifests with the formation of blood clots in atypical locations, such as the brain (known as cerebral venous sinus thrombosis) or abdomen, accompanied by decreased platelet counts. TTS is characterized by the occurrence of blood clotting alongside reduced levels of platelets, a condition known as thrombocytopenia.
It is believed that TTS may arise from an immune response elicited by the adenovirus vector utilized in these vaccines, leading to platelet activation and subsequent blood clot formation. Symptoms associated with TTS encompass severe headache, vision disturbances, speech difficulties, chest discomfort, abdominal pain, breathing difficulties, and swelling in the legs, among other indicators.
To read more topics, please visit: https://insightfulbharat.com