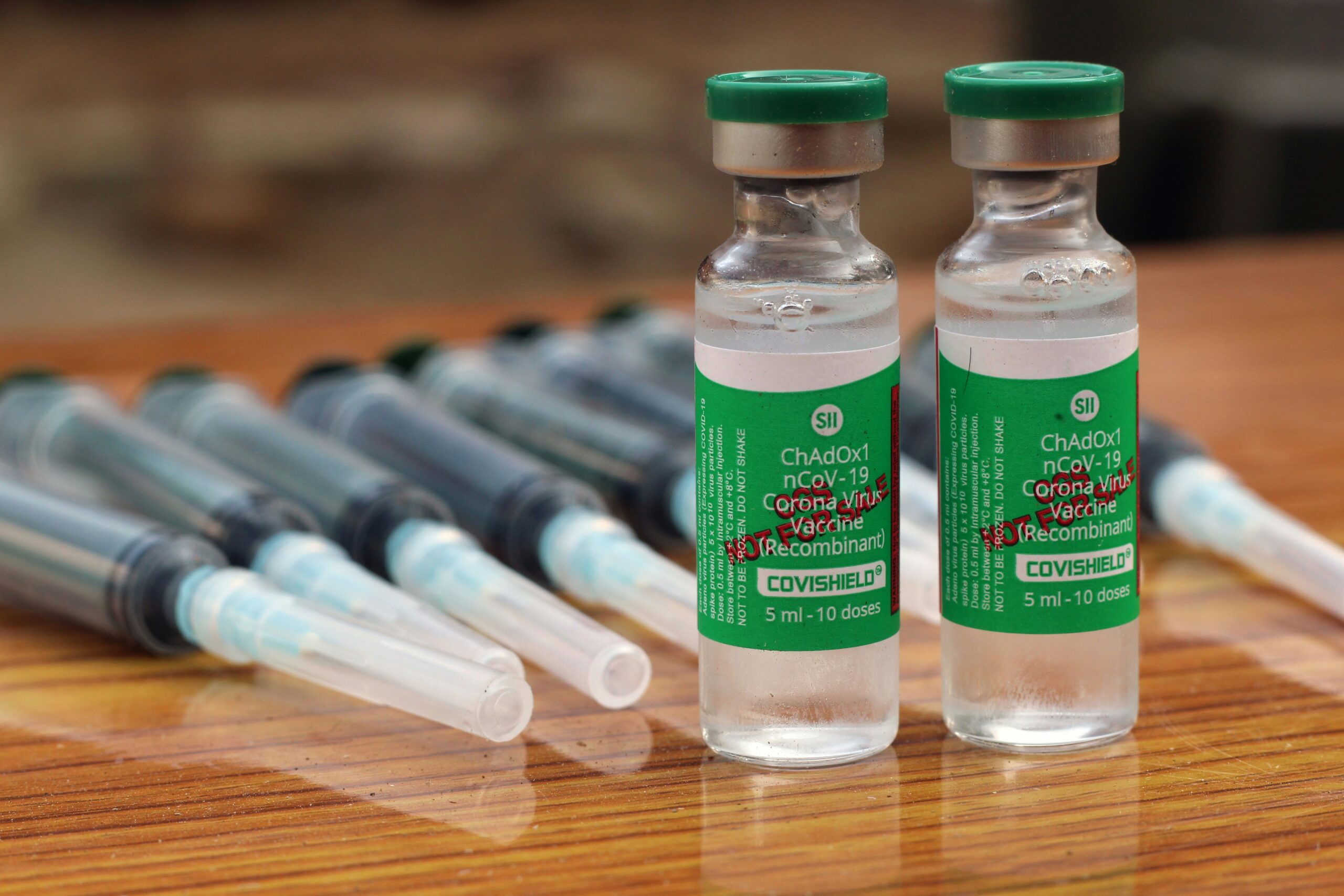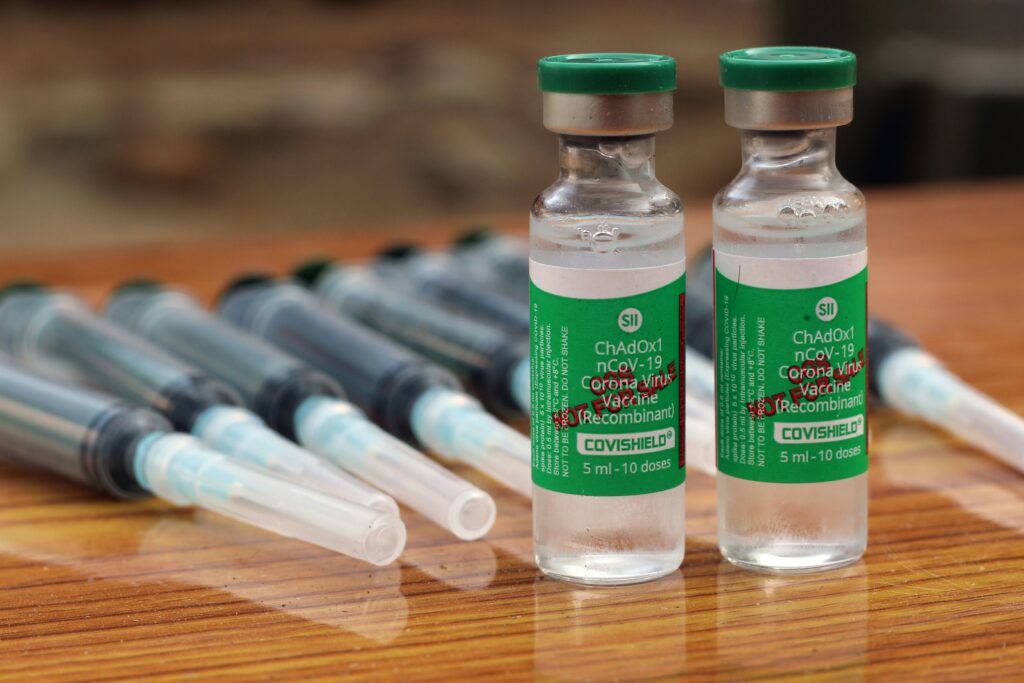
AstraZeneca Vaccine Scrutiny and Global Impact.
Hello, everyone! As you are well aware, in response to the emergence of the Coronavirus pandemic in India, the primary vaccine distributed to the population was CoviShield. This vaccine, produced by the Serum Institute of India under the licensing agreement with AstraZeneca, represents a significant milestone in the global fight against COVID-19. Developed through a collaborative effort between AstraZeneca and Oxford University, CoviShield quickly became a cornerstone of vaccination efforts worldwide. Despite its widespread usage, the CoviShield vaccine faced scrutiny in 2021, with several countries imposing restrictions on its distribution. This decision came amidst concerns regarding potential side effects and efficacy. Despite these challenges, CoviShield remained a critical tool in combating the pandemic, offering hope and protection to millions of individuals worldwide.
Search
Recent Posts:
- OpenAI Brings ChatGPT to be used in WhatsApp: Here’s How It Works and What You Can Do To Use It.
- Realme 14x 5G: A Budget Smartphone With Premium Features.
- Exploring Apple Genmoji: A New Era of Custom Emoji Creation.
- 2024 United States Presidential Election: Donald Trump Declares Victory in 2024 Presidential Election
- Chancellor Olaf Scholz’s Visit to India: Advancing Indo-German Cooperation on Defense, Trade, and Regional Stability.
AstraZeneca Admits Rare Side Effects.
In a recent development, AstraZeneca has acknowledged the potential for serious side effects associated with their vaccine. Specifically, the company has conceded that the vaccine may lead to rare blood clots, a revelation disclosed in a submission to the UK High Court in February. This rare side effect, known as Thrombosis with Thrombocytopenia Syndrome (TTS), underscores the complexity of vaccine-related complications. AstraZeneca emphasized that the occurrence of TTS may not be directly linked to vaccination and requires expert evaluation to ascertain causality in individual cases.
AstraZeneca has been embroiled in a class action lawsuit alleging that its Covid vaccine, developed in collaboration with the University of Oxford, has resulted in numerous fatalities and severe injuries. The unexpected onset of the Covid-19 pandemic in 2020 prompted a concerted effort in medical science to develop a vaccine. Despite the staggering loss of life worldwide, researchers achieved a seemingly insurmountable feat within a remarkably short timeframe by developing a vaccine, swiftly administering it to people globally.
Resurfacing Doubts.
However, skepticism arose within the scientific community regarding the expedited rollout of vaccines, with concerns raised about potential compromises in safety protocols and the durability of vaccine efficacy. These apprehensions have resurfaced amidst recent developments. Pune-based pharmaceutical giant Serum Institute of India (SII) entered into a strategic collaboration with AstraZeneca and the University of Oxford in January 2021 to manufacture the CoviShield vaccine for India and other low and middle-income countries. By 2022, over 1.7 billion doses of CoviShield had been administered in India as part of the world’s largest vaccination initiative. With its relatively straightforward storage requirements, the AstraZeneca vaccine has played a pivotal role in global vaccination campaigns.
Understanding the Rare TTS Syndrome Linked to Covid-19 Vaccines.
Thrombosis with Thrombocytopenia Syndrome (TTS) is a rare yet severe condition associated with certain Covid-19 vaccines, particularly adenovirus vector vaccines like the AstraZeneca and Johnson & Johnson’s Janssen vaccines. TTS manifests with blood clot formation in atypical sites such as the brain or abdomen, accompanied by low platelet counts.
The condition arises from an immune response triggered by the adenovirus vector, activating platelets and leading to blood clot formation. Symptoms of TTS include abdominal pain, blurred vision, leg swelling, chest pain, shortness of breath, severe headache, and difficulty speaking.
Following heightened scrutiny of the AstraZeneca vaccine, numerous countries imposed bans on its usage. Denmark was the first to suspend the AstraZeneca Covid-19 vaccine, with other countries like Ireland, Thailand, Netherlands, Norway, Iceland, Congo, and Bulgaria swiftly following suit. European nations including Germany, France, Italy, and Spain also halted the use of AstraZeneca’s vaccine in 2021 following reports of blood clotting in vaccinated individuals. Subsequently, Canada, Sweden, Latvia, and Slovenia joined the ban, expressing public concerns about vaccine safety. Despite these bans, the World Health Organization issued a statement reaffirming the safety of AstraZeneca’s Covid-19 vaccine and recommending its continued use, citing its benefits outweighing potential risks.
To read more topics, please visit: https://insightfulbharat.com